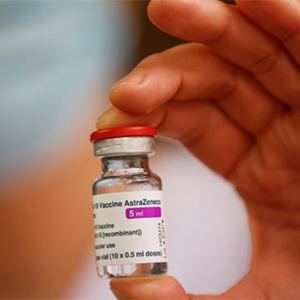
The Netherlands has become the latest country to suspend use of the Oxford-AstraZeneca coronavirus vaccine over concerns about possible side effects.
The World Health Organization and Europe’s Medicines Regulator say there is no indication of a link between the vaccine and reports of blood clots.
The Dutch government said the move, which will last until at least 29th March, was a precaution.
The Irish Republic took similar action over blood clotting reports in Norway.
Similar moves have been taken by Denmark, Norway, Bulgaria, Iceland, the Democratic Republic of Congo and Thailand.
Several other European countries, including Italy and Austria, have suspended the use of certain batches of the drug as a precautionary measure.
AstraZeneca said about 17 million people in the European Union and the UK have received a dose of the vaccine, with less than 40 cases of blood clots reported as of last week.
The European Medicines Agency (EMA) – which is currently carrying out a review into incidents of blood clots – said the vaccine could continue to be administered.
It said the incidents of clotting in vaccinated people were “no higher than the number seen in the general population”.
The UK medicines regulator also said evidence “does not suggest” the jab causes clots, as it urged people in the country to get the vaccine when asked to do so.





 LATEST ON
LATEST ON
Tweets by @i955fmTWITTER
FACEBOOK
This message is only visible to admins.
Problem displaying Facebook posts.
Click to show error